
Winter Is Coming:
The Changing Landscape of Head and Neck Cancer Biomarkers
Nadeem Riaz, MD, and Bhisham Chera, MD, FASTRO
In the Heat of the Bite
While it is true that the human papilloma virus (HPV) is sexually transmitted and leads to head and neck and other cancers, at least we aren’t plagued by the same malady suffered by Sarcophilus harrisii. Best known to children growing up in the U.S. by its Looney Tunes cartoon incarnation, the hapless Tasmanian Devil is susceptible to a facial sarcoma whose transmission is thought to be unique in the animal kingdom. Allografts are directly deposited by bites during a violent mating ritual.1
Devil Facial Tumor Disease is no laughing matter, though, and was considered a legitimate threat to species extinction. Fortunately, in recent years there has been the apparent development of resistance to transmission of the tumor, alongside Australian and Tasmanian government programs to maintain a captive insurance population. Also, vaccine tests are underway.2
REFERENCES
- Pearse AM, Swift K. Transmission of devil facial-tumour disease. Nature. 2006;439(7076):549-549.
- Conroy G. Tasmanian devil cancer vaccine approved for testing. Nature News. June 30, 2023. Accessed Nov 18, 2024. https://www.nature.com/articles/d41586-023-02124-4.
Treating head and neck cancer can be like a battle for the Iron Throne, and it’s not just because of the anatomical complexity or the sheer variety of histologies. No, the real chaos happens at the molecular level, where biomarkers are supposed to be guiding us but instead leave us feeling like we’re deciphering the words of a court jester, desperately searching for that magic marker that will bring order to heterogeneity. Let’s be honest, the promise of biomarkers has been sitting on the horizon for years now, but it’s time to take a critical look at what’s real and what’re just whispers in the shadows.
In the Beginning: p53, EGFR, EBV, HPV
The “first” biomarkers we had for head and neck cancer were p53 and EGFR — early rulers of a chaotic molecular realm. Disruptive p53 mutations and EGFR amplification/mutations served as grim heralds, prognostic for worse survival. The arrival of cetuximab, a monoclonal antibody targeting EGFR, was akin to the introduction of a promising new claimant to the throne — an aspirant that, despite early victories, ultimately after 20 years of iterative clinical trials could not unseat the enduring power of cisplatin. We subsequently discovered that uniquely many nasopharyngeal and oropharyngeal cancers are caused by viruses: Epstein Barr Virus (EBV) and Human Papilloma Virus (HPV), respectively.The discovery of HPV-related oropharyngeal cancers emerged as our “Prince that Was Promised” — a beacon of hope for personalizing our most toxic treatments. HPV is spread through intimate alliances and identified through p16 staining, an indirect marker linked to the viral oncoprotein E7. This discovery allowed us to recognize and predict excellent outcomes for these patients under standard treatment. However, over a decade of de-escalation trials — attempting to reduce RT dose or substitute cisplatin with cetuximab — has shown that a one-size-fits-all approach to de-escalated treatment remains out of reach in the standard of care setting.
Intra-Treatment Monitoring and ctDNA
While we discovered a new disease in HPV-related oropharyngeal cancer, we still need to apply greater precision to understand the biology of these tumors and better tailor our treatments. Intra-treatment response monitoring, using tools like circulating tumor DNA (ctDNA) or PET imaging, to identify early responding tumors for de-escalation, has shown promise in early studies. For instance, intra-treatment monitoring of tumor hypoxia with FMISO PET allowed a subset of patients to receive doses as low as 30 Gy, significantly less than conventional therapy.1 Patients with resolution of hypoxia two weeks into therapy received 30 Gy, while those who did not respond continued with standard treatment. Likewise, intra-treatment FDG PET response monitoring has enabled dose reductions to 54 Gy in responsive patients. Despite these promising results, we must be cautious not to repeat past mistakes; rigorous Phase 3 trials are needed before integrating these approaches into standard care.
Similar to imaging, ctDNA can identify rapidly responding tumors and may be able to de-escalate curative treatment in HPV related cancer. HPV related ctDNA (ctHPVDNA) is easy to detect and widely commercially available today. The intuitive assumption is that ctHPVDNA might be a valuable treatment-personalizing tool by revealing which patients have microscopic disease after surgery and thus need adjuvant therapy. However, early studies suggest these tests may not be sensitive enough today for that purpose.2 Another emerging use for ctHPVDNA is in the post-radiation treatment surveillance setting. Here, a positive ctHPVDNA test often indicates a high risk of recurrence, necessitating closer surveillance.3,4 Although not yet included in consensus guidelines, some routinely incorporate ctHPVDNA in this setting. Data suggest that recurrence can be detected in an early state with this approach. Figure 1 illustrates the possible value of ctHPVDNA across the continuum of care for a patient with HPV related oropharyngeal cancer.
EBV viral load is a standardly used biomarker for nasopharyngeal cancer. It is not as sensitive or specific as ctHPVDNA because the viral load assay does not provide absolute quantification and cannot distinguish between cancer and acute infection (i.e., mononucleosis) because it is detecting the intact viral genome. Nonetheless, pretreatment, mid-treatment and post-treatment EBV viral load is prognostic, and consensus guidelines suggest EBV monitoring.
Biomarkers at Recurrence
If a cancer returns, we possess several biomarkers to guide our counterattack. The most important of these is PD-L1, an immune checkpoint that can guide decisions on first line therapy in the recurrent/metastatic setting. The Combined Positive Score (CPS) for PD-L1 is complex, counting tumor cells, immune cells and other elements, but it helps identify patients who may respond to pembrolizumab alone, sparing them from combination chemotherapy. Next-generation sequencing can identify actionable alterations in HNSCC, including PI3KCA and H-ras mutations. Unfortunately, unlike our colleagues in lung, prostate and breast cancer, we do not yet have FDA-approved targeted therapies for these alterations, leaving us to recommend clinical trials instead. Similarly, more detailed HPV typing, distinguishing HPV16 from other subtypes, can provide unique trial opportunities for patients.
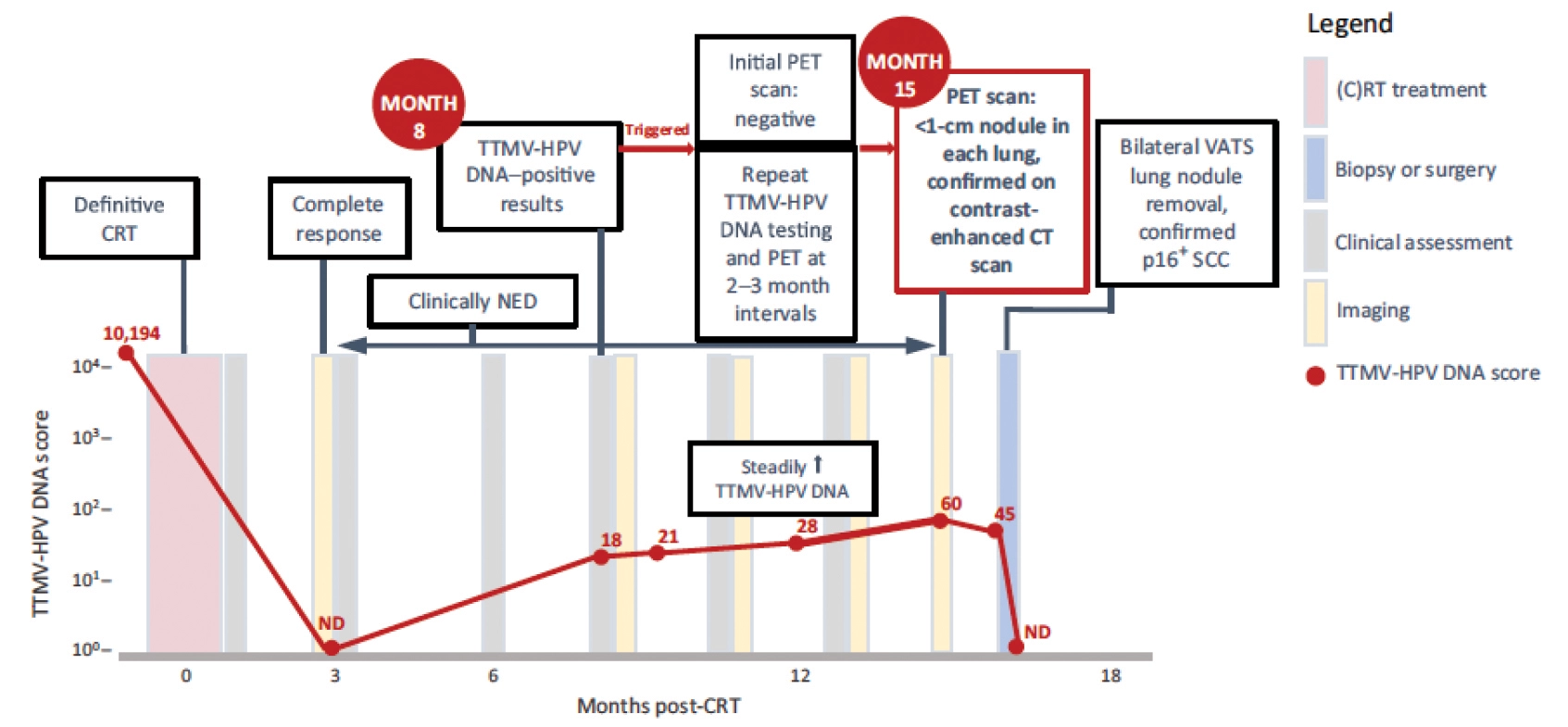
Figure 1: Adapted from Berger et al.4 Patient with p16 positive oropharyngeal squamous cell carcinoma received definitive chemoradiotherapy resulting in complete response and complete resolution of ctHPVDNA (TTMV-HPV DNA). Eight months post treatment ctHPVDNA became detectable which triggered a repeat PET/CT which showed no evidence of disease. Serial ctHPVDNA steadily increased and repeat PET/CT 15 months post-treatment revealed oligometastatic disease in the lung which was treated with surgery. ctHPVDNA subsequently became undetectable. TTMV-HPV DNA = Tumor Tissue Modified Viral HPV DNA.
Closing Out
The next time you’re tempted to put your hopes on the latest biomarker buzz, remember that the head and neck cancer landscape is as treacherous as the battle for the Seven Kingdoms. We need more than just intriguing signals. We need reliability, reproducibility and relevance to real-world outcomes. Until we reach that goal, let’s keep our eyes open, our minds skeptical, and our focus on what truly matters: delivering the best, evidence-based care for our patients, regardless of how trendy the biomarker may be. Because in the end, it’s not just about winning the “Game of Thrones” — it’s about who survives to see another day.
References
- Lee NY, Sherman EJ, Schoder H, et al. Hypoxia-Directed Treatment of Human Papillomavirus-Related Oropharyngeal Carcinoma. J Clin Oncol. 2024 Mar 10;42(8):940-950.
- Chen L, Cohen M, Hatzoglou V, et al. Early Disease Recurrence Following Post-operative HPV ctDNA Directed Active Surveillance in Oropharyngeal Carcinoma – Outcomes of a Prospective Pilot Study. Int J Radiat Oncol Biol Phys. 2024;118(5).
- Hanna GJ, Roof SA, Jabalee J, et al. Negative Predictive Value of Circulating Tumor Tissue Modified Viral (TTMV)-HPV DNA for HPV-driven Oropharyngeal Cancer Surveillance. Clin Cancer Res. 2023;29(20):4306-4313.
- Berger BM, Hanna GJ, Posner MR, et al. Detection of Occult Recurrence Using Circulating Tumor Tissue Modified Viral HPV DNA among Patients Treated for HPV-Driven Oropharyngeal Carcinoma. Clin Cancer Res. 2022;28(19):4292-4301.




