Beyond the Tangents:
Biomarkers and the Art of Breast Radiotherapy
Chelain R. Goodman, MD, PhD
It wasn’t too long ago that many residents considered their rotation on the breast service to be the “easy” rotation. Write a prescription for 15 or 25 fractions, contour the tumor bed, throw on some tangents, and call it a day! If you’re particularly detail-oriented, you could consider contouring a couple of the lymph node basins — but anatomical landmarks, clips and wires were still considered the mainstay for beam placement.
I was therefore surprised when one of my residents admitted he was the most nervous about his upcoming breast rotation. With expanded options for treatment decisions (omission, partial breast, whole breast, “high tangents,” comprehensive regional nodal irradiation), dose fractionations (26Gy/5Fx QD, 30Gy/5Fx QD/QOD, 40Gy/15Fx, 50Gy/25Fx, 51Gy/34Fx BID), boosts (tumor bed, scar, flaps, nodal basins), and treatment technique (Partially Wide Tangents, Photon/Electron Match, VMAT, Sequential versus SIB Boost) — phew, I’m tired even writing all of these! — it’s no wonder my resident feels like it’s hard to read my mind.
Alongside this diverse menu of treatment opportunities has emerged a bevy of biomarkers with the potential to guide every aspect of breast radiotherapy: patient selection, dose prescription, target volume delineation, and even prevention and management of treatment-related toxicity. And we’re not just talking about the standard panel of immunohistochemical receptors (the original “Three Musketeers”: ER, PR, Her2) from the old days. Remember when Ki-67 was the hot new thing? There is now a bewildering array of assays that promises to transform breast radiotherapy from a “one-size-fits-most” approach to customized treatment that is as individual as the patient themself. The following is a select sample of my favorite biomarkers with the potential to move the needle in patient care:
Genomic Classifiers for Risk Stratification: “Oncotype, ARTIC and POLAR, Oh My!”
Genomic assays such as OncotypeDx, Mammaprint and Prosigna have been increasingly utilized to guide chemotherapy decisions for patients with early-stage breast cancer. But these tests aren’t just limited to systemic therapy decisions; clinical trials currently underway propose a role in guiding radiotherapy decisions as well. While the Oncotype Dx and Prosigna assays are under evaluation as risk stratification biomarkers for radiotherapy de-escalation (e.g., TAILOR RT and PRECISION trials), conceptual frameworks such as POLAR (Postoperative Radiation for Low-Risk Patients) and ARTIC (Adjuvant Radiotherapy for Intermediate-Risk Cases) represent the next wave of risk stratification. Unlike the one-size-fits-all approach of yesteryear, these frameworks incorporate clinical, pathologic and genomic data to optimize radiotherapy treatment decisions for individual patients.
“EnGARD!” Utilizing Genomics to Personalize Radiation Dose
Meanwhile, GARD (Genomic Adjusted Radiation Dose) is competing to bring radiotherapy into the genomic age beyond the limitations of risk stratification alone. Transcending reliance on clinicopathologic data alone to guide dose prescriptions, GARD builds on the Radiosensitivity Index (RSI) to calculate the biological effectiveness of a prescribed radiation dose based on a tumor’s genomic profile. Lacking at the moment from the breast radiotherapy armamentarium is the equivalent of p16 status for head and neck cancer, which may inform a more nuanced approach to dose prescription. This developing story will be exciting to follow in the coming years.
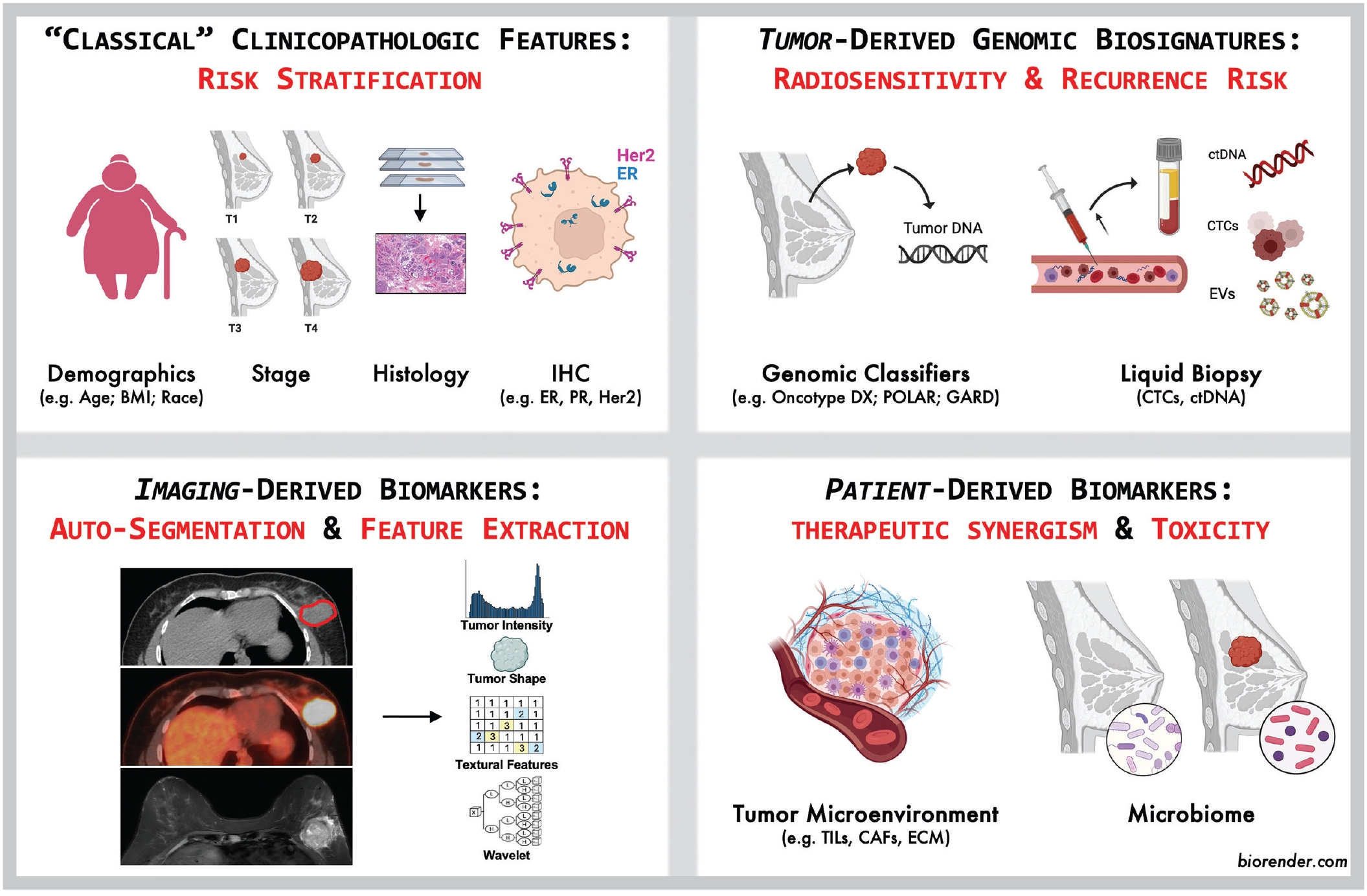
Circulating Biomarkers: Liquid Gold?
Circulating tumor material, including circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA), has been likened to a microscopic “crystal ball” for oncologists, providing a real-time glimpse into tumor dynamics and mutational changes with treatment. In his commentary in this issue of ASTROnews, Scott Bratman, MD, PhD, introduced the notion of minimal residual disease (MRD), defined as the presence of circulating tumor material after definitive treatment. Dozens of studies (and counting) have explored the use of MRD in breast cancer to yield prognostic information or even potentially guide management decisions, and it seems we are approaching a time when this type of assay will add value.1 We are not yet, however, at a point where a ctDNA assay is an established instrument to direct therapy for breast cancer — but stay tuned.
“Always Look for the Helpers”: The Tumor Microenvironment
Far more than just a bystander, the tumor microenvironment (TME) may be the true “shadow player” behind treatment outcomes. Consisting of cancer-associated fibroblasts, extracellular matrix components, lymphovascular networks and immune cells, the TME is a complex ecosystem that can modulate tumor radiosensitivity, promote anti-tumor immunity and facilitate (or inhibit) the epithelial-mesenchymal transition associated with distant metastatic spread. An “easy” example of the relevance of the TME is the fact that immune checkpoint inhibitors now play an important role in the management of triple negative breast cancer, but there is reason to believe that additional therapies might eventually exploit other features of the TME.2 Radiation certainly has myriad effects on the TME, which has been labeled “game changer” for radiotherapy.3 But at the moment, it seems we are still figuring out the rules.
Say “Cheese”: The Wonderful World of Quantitative Imaging Biomarkers
The extraction and analysis of quantitative imaging biomarkers can provide insights into tumor biology and radiosensitivity that transcends what may be visible to the human eye. Multiparametric quantitative imaging analysis of tumor heterogeneity can identify microstructural patterns associated with radioresistance, while extraction of features correlating with tumor subtype, proliferation index or genomic changes have the potential to serve as a longitudinal supplement or surrogate for invasive biopsies. Investigators are just now scratching the surface in this domain.4
Don’t Forget the Patient! Host Biomarkers and Toxicity Prediction
While much of the focus on biomarkers remains on tumor biology and oncologic outcomes, we would be remiss to ignore host-related biomarkers in determining the risk of radiotherapy treatment-related toxicity. Germline mutations in genes like TP53, ATM and TGFB1 may increase sensitivity to radiation and the risk of late toxicities, while inflammatory biomarkers and cytokine profiles are gaining traction for predicting acute and long-term toxicities such as dermatitis, radiation fibrosis and lymphedema. Validation of biomarkers and probabilistic models for treatment-related toxicity would serve as a critical rationale for the development of prophylactic or proactive interventions to improve quality of life without compromising treatment efficacy.
Not All Fun and Games: Challenges Ahead
Alas, biomarker development comes with its own meaningful set of challenges. The validation of biomarkers for clinical or regulatory use requires large prospective clinical trials, while integration into clinical practice demands robust infrastructure. Importantly, we must consider whether biomarker-driven advances will prove to exacerbate disparities in access to care and subsequently address identified barriers to equitable access. Lastly, while biomarkers offer the potential for unprecedented precision, their effectiveness is only as good as the physician integrating these assays into their clinical practice. As we embrace new treatment tools, maintaining a holistic view of the patient and continuously engaging in patient-centered decisions will remain critical.
The Bottom Line for Breast Biomarkers
As we stand on the brink of this biomarker revolution, one thing is clear: the future of breast radiation oncology is not just delivering Gray to kill. With the advent of biomarkers derived from genomic classifiers, circulating tumor material, quantitative imaging parameters, the tumor microenvironment and microbiome, radiation treatment continues to evolve into an ever more personalized art form. For radiation oncologists, the challenge will be not only staying abreast of these advances but also weaving them effectively into the fabric of day-to-day clinical practice.
So, whether you’re a senior FASTRO, a seasoned community radiation oncologist, a stressed-out junior faculty, or an idealistic resident, one thing seems to be clear: breast radiotherapy is no longer the “easy” rotation — but it might just be the most personalized!
References
- Pantel, K and Alix-Panabières, C. Minimal residual disease as a target for liquid biopsy in patients with solid tumours. Nat Rev Clin Oncol. 2025;22(1):65–77.
- Harris, MA et al. Towards targeting the breast cancer immune microenvironment. Nat Rev Cancer. 2024;24(8):554-577.
- Jarosz-Biej, M, Smolarczyk, R, Cichoń, T. et al. Tumor Microenvironment as A “Game Changer” in Cancer Radiotherapy. IJMS. 2019;20(13):3212.
- Llorián-Salvador, Ó. et al. CT-based radiomics for predicting breast cancer radiotherapy side effects. Sci Rep. 2024;14(1):20051.


