

New this year! ASTRO will feature a Contouring Case of the Day. Each day of the meeting, join us in reviewing a new case. Each case will include a case description and four possible scenarios with the question "How would you contour for this patient?"
Following each day's Contouring Case of the Day, an answer and rationale will be given by the presenter of that case. Would you have contoured the same way?
| Schedule | Case | Faculty* |
|---|---|---|
| Sunday, September 29 | Pancreas | Karyn Goodman, MD, MS, FASTRO - Icahn School of Medicine at Mount Sinai Leila Tchelebi, MD - Northwell Health |
| Monday, September 30 | Lung | Raymond Mak, MD - Dana-Farber/Brigham Cancer Center |
| Tuesday, October 1 | Head and Neck | Sung Kim, MD - Rutgers Cancer Institute of New Jersey |
| Wednesday, October 2 | Breast | Mylin Torres, MD, FASTRO - Emory University |
*Special thanks to Stanley Liauw, MD, FASTRO, and Salma Jabbour, MD, FASTRO, in helping to prepare the cases.
Faculty
Mylin Torres, MD, FASTRO – Emory University
A 44-year-old premenopausal woman self-palpated left lateral breast mass. Biopsy reveals invasive ductal carcinoma, grade 3, ER+/PR-, Her2 3+. MRI and PET show a 1.4 cm axillary node which is biopsy confirmed disease. She has no distant metastatic disease. She is treated with TCHP (taxotere, carboplatin, trastuzumab, pertuzumab) x 6 cycles, and lumpectomy with sentinel lymph node biopsy shows complete pathologic response in the breast and all 5 lymph nodes.

Which clinical target volume contours are the most appropriate?
Expert Commentary
Answer: D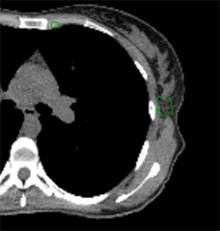 | 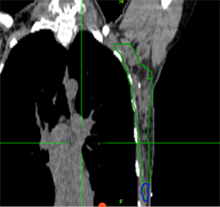 | 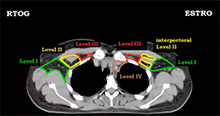 |
Review the case below and let us know on X and LinkedIn how you would contour this case. Come back tomorrow for the expert’s commentary on how the case was contoured.
Faculty
Sung Kim, MD – Rutgers Cancer Institute of New Jersey
A 70-year-old man with no smoking history presented with a right neck mass. FDG PET showed uptake in the tonsil and the neck at level II. Fine needle aspiration of the neck mass revealed p16+ squamous cell carcinoma (SCCA). He underwent transoral robotic surgery; intra-operative exam showed minimal base of tongue involvement and no soft palate invasion. Pathology after tonsillectomy and ipsilateral neck dissection revealed a 3 cm SCCA primary tumor, closest margin 3 mm, without perineural invasion or lymphovascular space invasion. The level II node was 4 cm without extranodal extension, pT2N1.
In terms of treating the neck, which levels would you radiate?

Expert Commentary
Answer: B
The indication for adjuvant radiation in this case was a lymph node > 3 cm. The primary was T2 without PNI or LVSI and 3 mm margins, so I did not specifically target the primary, though I did include some clips. The treatment I chose for this patient was option B, treating the right level II-V (56 Gy) plus the retrostyloid and lateral retropharyngeal lymph nodes (50 Gy). Chemotherapy was not indicated. Simply treating II-V would not have been adequate, as oropharyngeal primaries do drain to the retropharyngeal lymph nodes, and failure there can be catastrophic. Furthermore, with a 4 cm ipsilateral lymph node in level II, there is risk of retrograde drainage to the retropharyngeal region.
The risk of failure in the contralateral neck is driven by the size and laterality of the primary and ipsilateral neck tumor burden. In this case, the contralateral neck was not treated because the primary was small and well lateralized, with minimal invasion into the base of tongue or soft palate. With an ipsilateral 4 cm level II lymph node, the risk of failure in the contralateral neck is likely to be low. Sparing the contralateral neck reduces dose to the contralateral parotid, submandibular gland, and pharyngeal constrictor muscles. In my practice, I would consider coverage of the contralateral neck for multiple, bulky nodes (e.g. a few 4 cm lymph nodes).
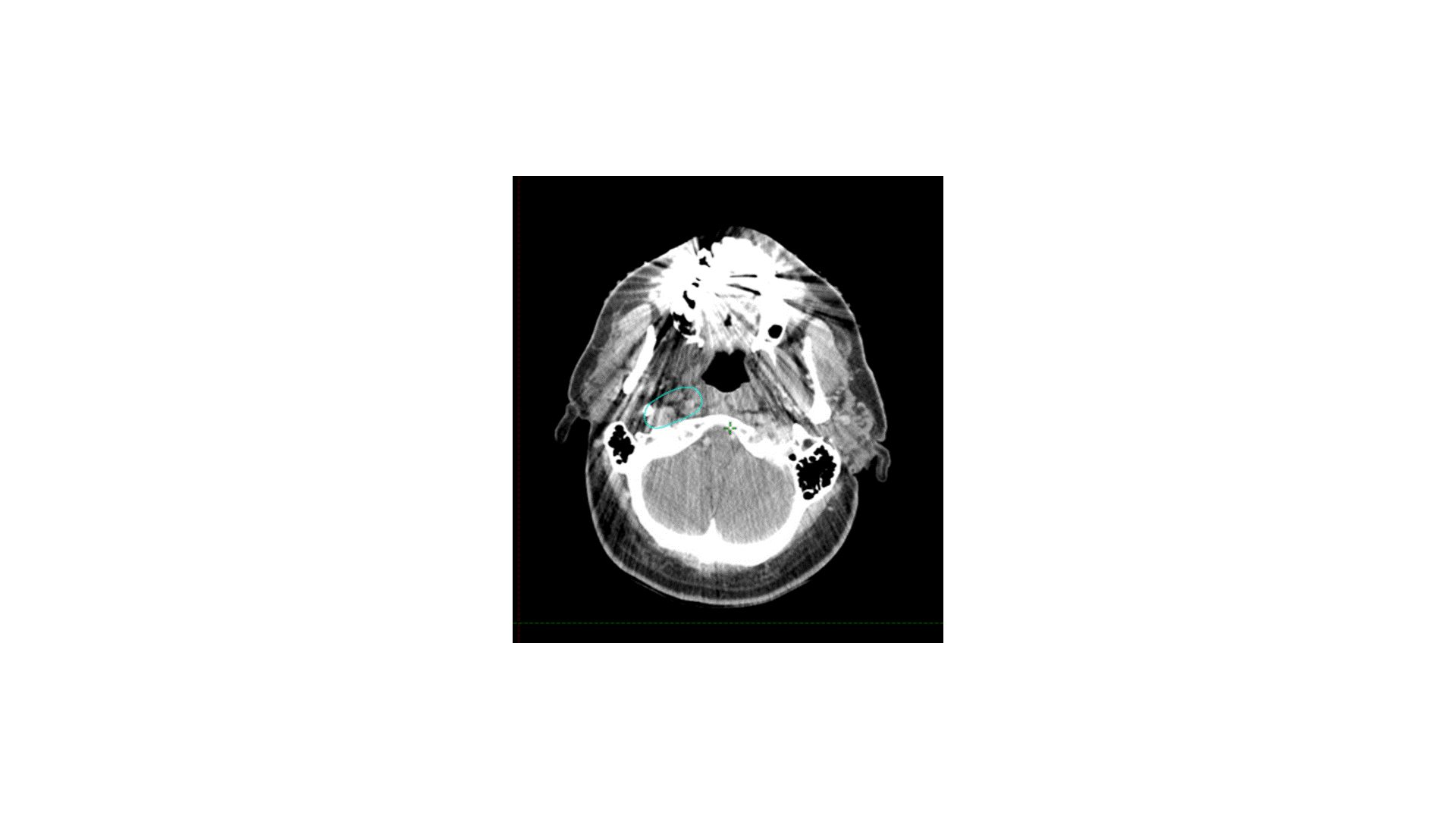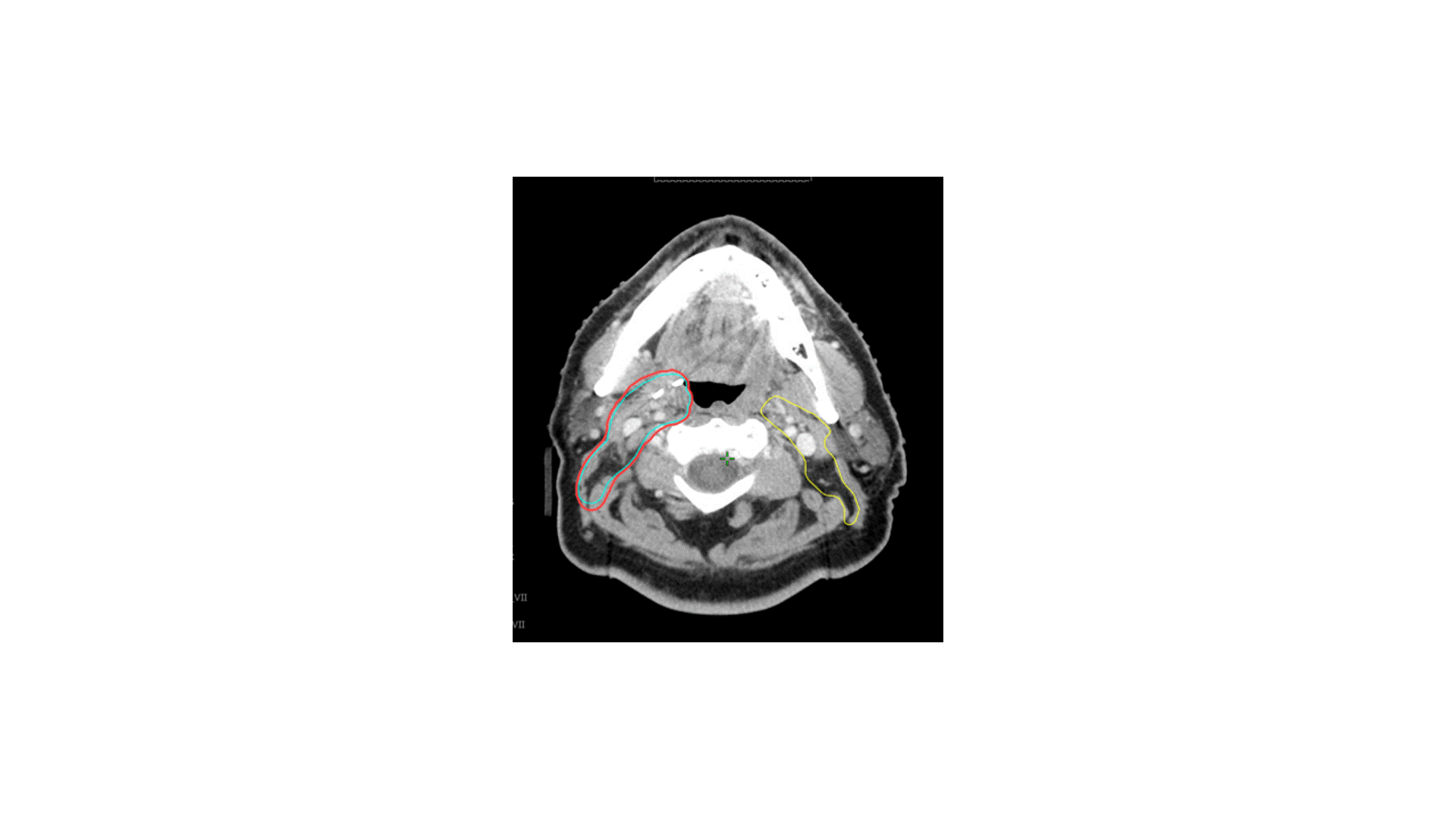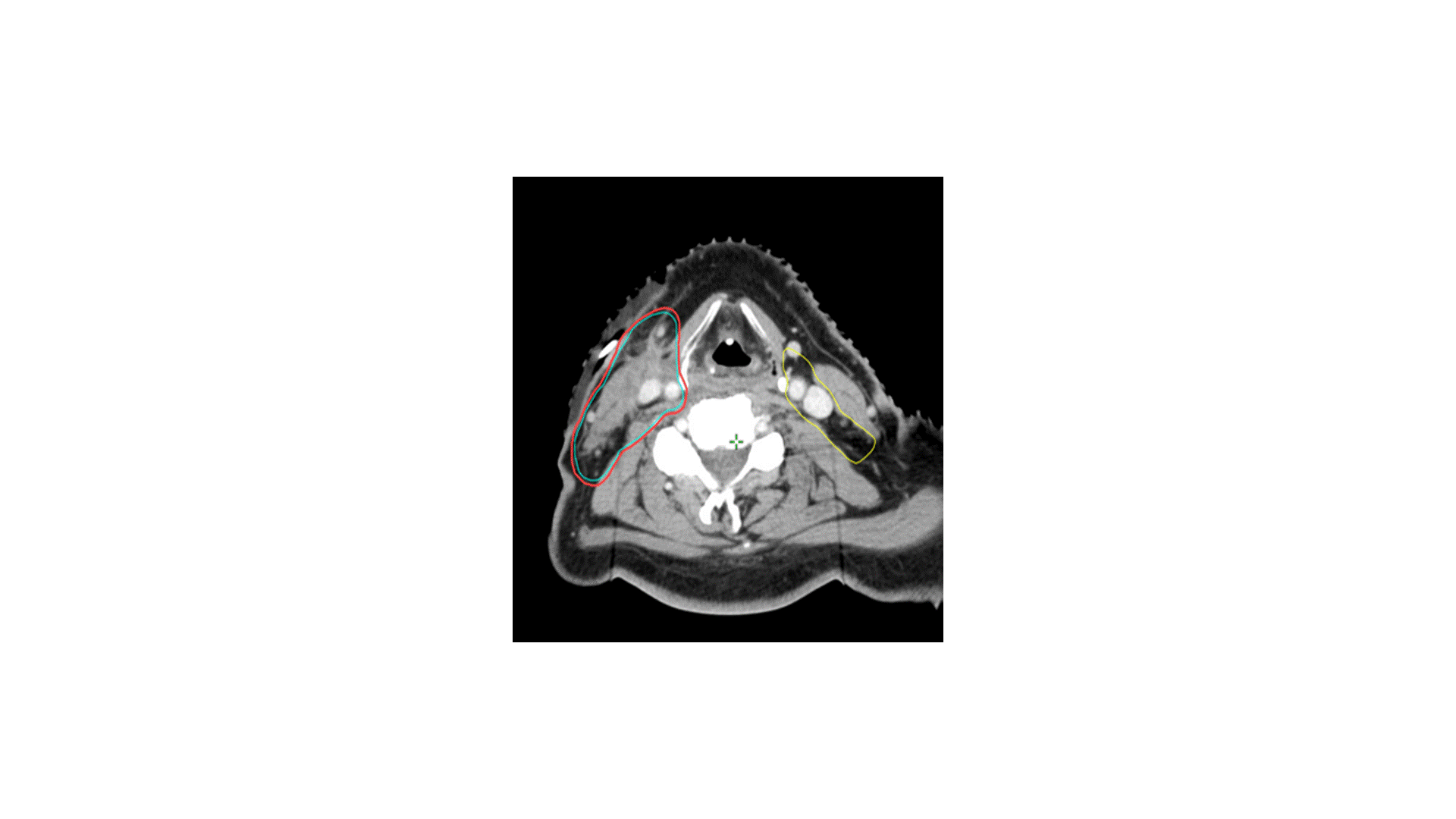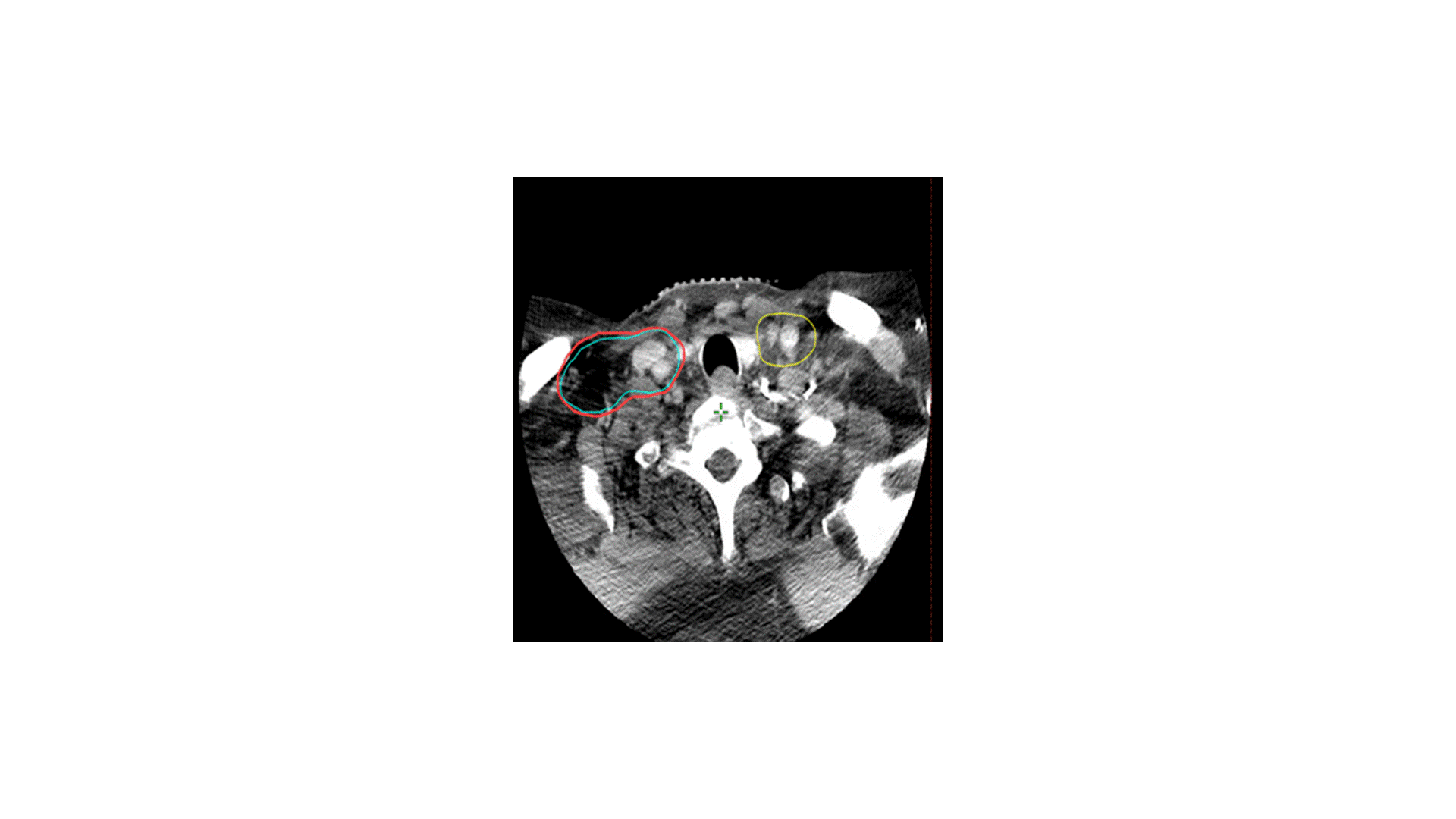
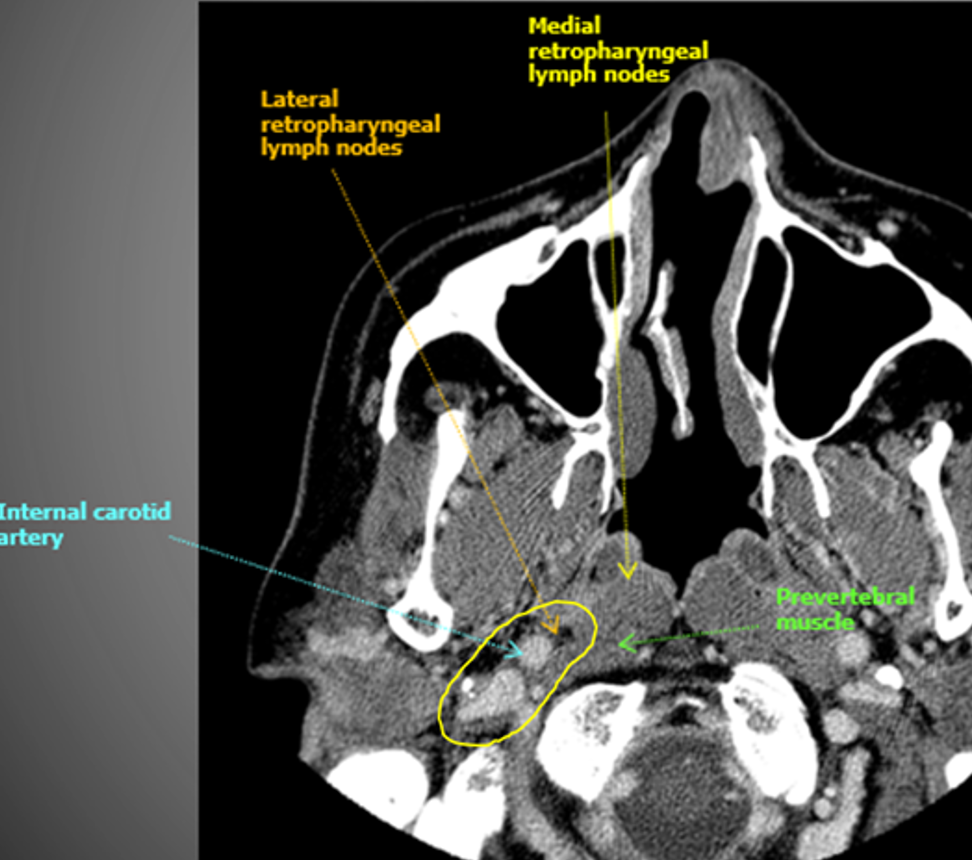
How I do it: defining retropharyngeal nodes at risk
Remember that there are lateral and medial retropharyngeal lymph nodes (RPLNs). When there is pathologic involvement of RPLNs, it is almost always the lateral RPLN that is involved first. Note that the lateral RPLN space is the fat space (dark on CT) immediately medial to the internal carotid artery.
When electively treating the retrostyloid and RPLN as in this case, I usually only treat the lateral RPLN. If the RPLN was grossly involved with cancer, I would include both the lateral and medial RPLNs.
Faculty
Katelyn Atkins, MD, PhD – Cedars-Sinai Medical Center
Gerard Walls, FRCR, PhD – Patrick G. Johnston Centre for Cancer Research
Raymond Mak, MD – Dana-Farber/Brigham Cancer Center
78-year-old male with a 30 pack-year history of smoking and previously diagnosed with Stage IIIA (T4 N0 M0) non-small cell lung carcinoma status post RUL/RLL bilobectomy two years ago and subsequently developed biopsy proven recurrence in right hilar and subcarinal lymph nodes. Definitive chemoradiation to 60 Gray with outback durvalumab is recommended after multi-disciplinary discussion.
The patient has no known history of cardiac disease, but the presence of coronary calcium is noted on the planning CT scans (4D-CT without contrast and free-breathing CT with contrast).
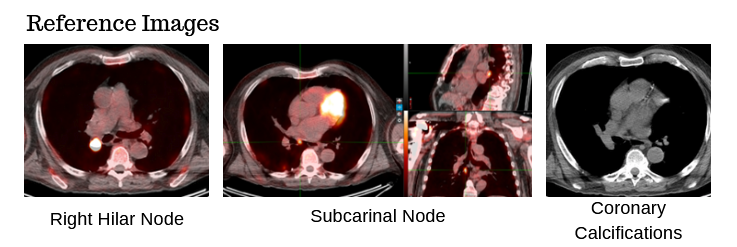
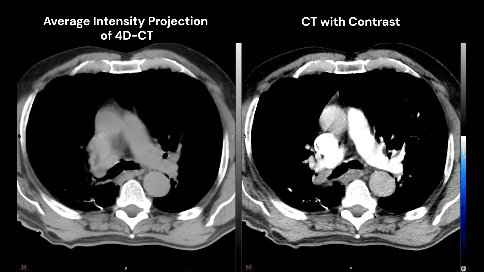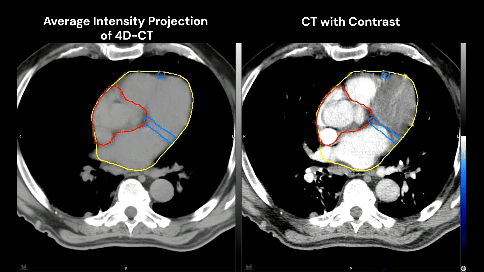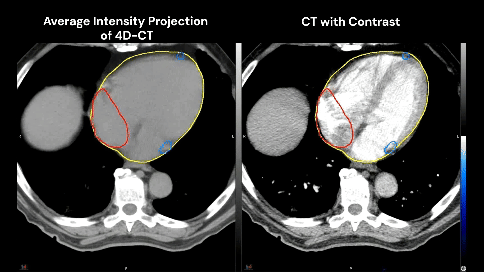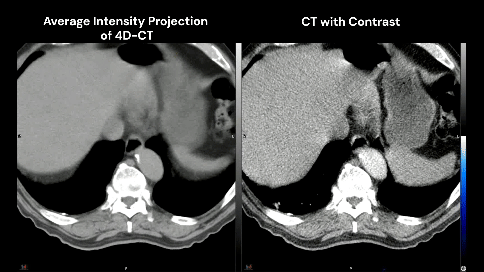
Which cardiac contour(s) would be most appropriate for planning?
Expert Commentary
Thank you for participating in Monday's Contouring Case of the Day. Below is the expert commentary with the answer for the pancreatic cancer case.
There is growing awareness of the impact of cardiac sub-structure radiation dose exposure on the risk of cardiac injury in lung cancer patients. Historically, the whole heart has been contoured, and the standard of care (such as NCCN guidelines) remains to constrain the whole heart (e.g. mean heart dose < 20 Gy) in the setting of definitive RT for lung cancer. Whole heart dose has been associated with survival (e.g. RTOG 0617), but a survival endpoint is multi-factorial and does not fully capture the cardiac impact of thoracic RT [1]. Emerging data suggests cardiac sub-structure radiation exposure may be more highly associated with specific cardiac toxicity events. For example, intermediate dose to the left-sided coronary arteries (e.g. V15) has been associated with the risk of coronary-related toxicity (e.g. major cardiovascular adverse events including MI, heart failure, coronary stenosis requiring intervention, and sudden cardiac death), while pulmonary vein dose has been associated with the risk of atrial fibrillation [2–5]. Thus, the ability to contour cardiac sub-structures at risk will be an important skill set for lung cancer RT moving forward.Faculty
Karyn Goodman, MD, MS, FASTRO - Icahn School of Medicine at Mount Sinai
Leila Tchelebi, MD - Northwell Health
Salma Jabbour, MD, FASTRO, Rutgers Cancer Institute of New Jersey
A 56-year-old female with clinical T2N0M0 adenocarcinoma of the head of the pancreas underwent pancreatic resection. Preop CA 19-9 was 153. Final pathology revealed pT2N0 disease with PNI. Negative margins. Postop CA 19-9 was 49.

Which clinical target volume contours are the most appropriate?